TNF Inhibitor Cancer Risk Calculator
Understand Your Risk
This tool compares cancer risk profiles of TNF inhibitors and alternative treatments based on current research. Results are simplified estimates for educational purposes only.
When you’re living with rheumatoid arthritis, psoriatic arthritis, or Crohn’s disease, the idea of taking a drug that suppresses your immune system can feel terrifying. You’ve heard the warnings: biologics might raise your cancer risk. But is that true? And if so, how big is the risk - and which drugs are actually involved?
Let’s cut through the noise. TNF inhibitors - a type of biologic drug - have been used for over 25 years to treat chronic autoimmune diseases. They work by blocking tumor necrosis factor-alpha, a protein that drives inflammation. For millions of people, these drugs have meant fewer flare-ups, less joint damage, and the ability to return to normal life. But the question of cancer risk has never gone away.
What Are TNF Inhibitors, and Who Uses Them?
TNF inhibitors are not your average pills. They’re complex proteins made in labs, given by injection or IV, and require refrigeration. Five are approved in the U.S.: infliximab, etanercept, adalimumab, certolizumab pegol, and golimumab. Together, they’re used by about 1.5 million Americans. Most are prescribed for rheumatoid arthritis, but they also help with psoriasis, ankylosing spondylitis, and inflammatory bowel disease.
They’re not all the same. Adalimumab and infliximab are monoclonal antibodies - they latch onto TNF like a key in a lock. Etanercept is a fusion protein that acts like a decoy, soaking up excess TNF before it causes trouble. Certolizumab is a smaller fragment, designed to penetrate tissues more easily. These differences matter - not just for how they work, but for how they affect cancer risk.
Doctors usually start these drugs after traditional treatments like methotrexate fail. The payoff is real: 50 to 70% of patients see at least a 20% improvement in symptoms within six months. But the cost? Around $62,000 a year. And while biosimilars have lowered prices, they’re still expensive. That’s why the risk-benefit calculation has to be sharp.
The Cancer Risk Debate: What the Data Really Shows
Back in 2012, a big study in JAMA raised alarms. It found that monoclonal antibody TNF inhibitors - like adalimumab and infliximab - were linked to a nearly threefold higher risk of cancer. Etanercept? No increase. That study changed how many doctors thought about these drugs.
But that was a short-term analysis of clinical trials. Real-world data tells a different story.
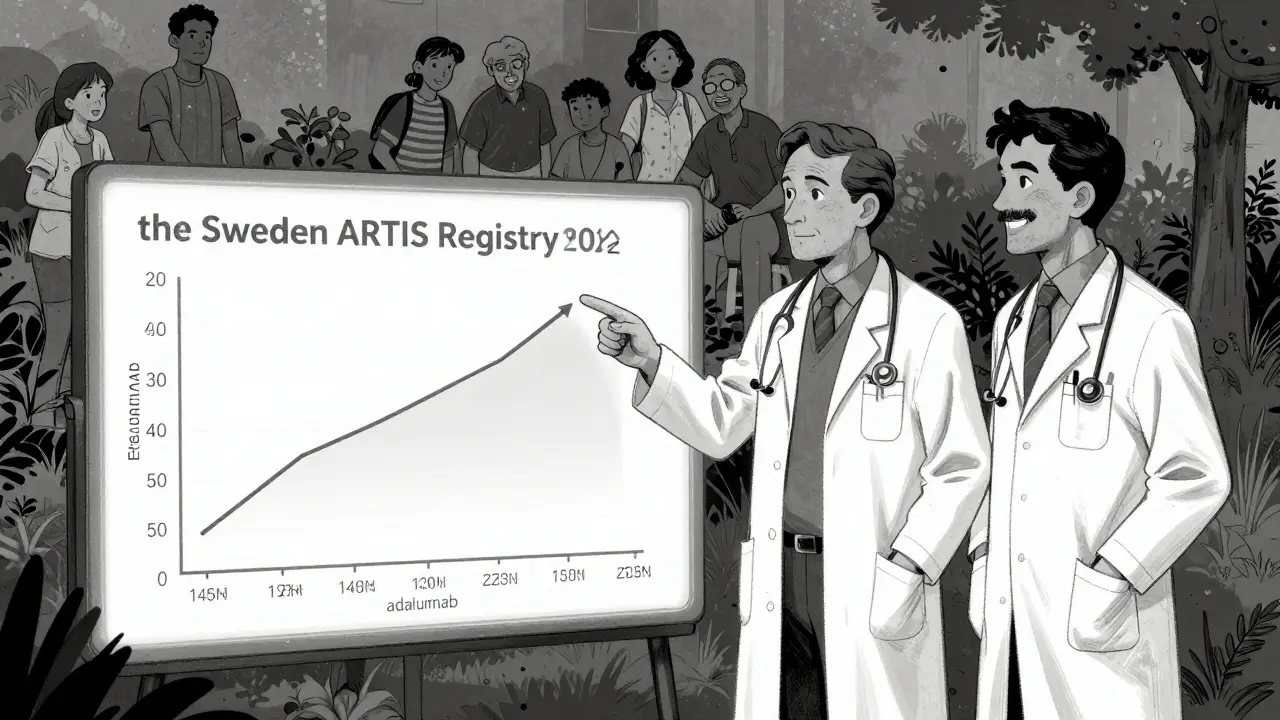
The 2022 Swedish ARTIS registry followed over 15,000 rheumatoid arthritis patients for up to 12 years. The result? No overall increase in cancer risk for anyone on TNF inhibitors compared to those on older drugs like methotrexate. The hazard ratio was 0.98 - essentially zero difference. That’s not a fluke. It’s a massive dataset, tracking real people over a long time.
But here’s the twist: adalimumab showed a small spike in cancer risk during the first year of treatment. That doesn’t mean it causes cancer. It might mean that people who were already developing cancer - undiagnosed - were more likely to start the drug because their symptoms got worse. That’s called protopathic bias. It’s not the drug causing cancer. It’s cancer causing the need for the drug.
Meanwhile, etanercept consistently shows lower or even protective trends in cancer risk. One study found patients on etanercept had a 22% lower risk of developing cancer than those never on biologics. Why? We don’t fully know. Maybe it’s the way it binds to TNF. Maybe it doesn’t interfere with immune surveillance the same way.
What About Skin Cancer?
If you’re on a TNF inhibitor and you have psoriasis, you’re already at higher risk for skin cancer. That’s because psoriasis itself is linked to chronic inflammation and sun exposure from light therapy. But does the drug make it worse?
A 2021 meta-analysis of over 32,000 psoriasis patients found a 32% higher rate of non-melanoma skin cancer - mostly basal cell and squamous cell carcinomas - in those on TNF inhibitors. That’s not nothing. But the risk for melanoma? No increase. No increase in lung, breast, colon, or lymphoma either.
And here’s something important: the risk isn’t the same across all drugs. A 2021 British Journal of Dermatology review found that adalimumab carried a 1.3 times higher risk of non-melanoma skin cancer than etanercept. That’s a real difference. If you’ve had skin cancer before, your dermatologist might push you toward etanercept.
Real-world experience backs this up. In a 2022 analysis of 478 patients on Reddit, 63% worried about skin cancer. About 28% reported being diagnosed with a basal cell carcinoma while on treatment. But here’s the good part: nearly all of them had the cancer removed early, with no spread. Regular skin checks - every six months - made the difference.
What If You’ve Had Cancer Before?
This is the hardest question. If you’ve had breast cancer, colon cancer, or melanoma - can you still take a TNF inhibitor?
Guidelines say: wait. For high-risk cancers like melanoma or lymphoma, wait five years after treatment. For low-risk cancers like early-stage breast or prostate cancer, two years is often enough. But these are just rules of thumb.
Real data shows something surprising. In the Corrona registry, 87% of rheumatologists continued TNF inhibitors in patients with early-stage solid tumors after consulting with oncologists. And 92% of those patients had no cancer recurrence linked to the drug.
One 2023 study looked at 1,872 RA patients who also had lung cancer. Those on TNF inhibitors had a 42% lower chance of dying within five years than those on older drugs. Why? Possibly because controlling inflammation helps the body fight cancer. TNF isn’t just bad - it can help tumors grow.
Dr. Paul Nguyen from Dana-Farber says: “We’re learning that suppressing TNF in someone with early-stage cancer might not be dangerous - it might even help.” That’s a radical shift from ten years ago.
What About Other Drugs? Steroids and JAK Inhibitors
It’s not just TNF inhibitors. Steroids like prednisone are known to increase cancer risk - especially if you’re on more than 7.5 mg a day. Studies show steroid users have 1.75 to 2.91 times worse cancer survival rates. That’s higher than any TNF inhibitor risk.
And now there are newer drugs: JAK inhibitors like tofacitinib and baricitinib. They’re oral, convenient, and effective. But the FDA has issued black box warnings for increased risk of serious infections, blood clots, and cancer - especially in people over 50 with heart disease. In some studies, JAK inhibitors showed higher lymphoma rates than TNF inhibitors.
So while TNF inhibitors get blamed, they might actually be safer than the alternatives. The 2024 global market report predicts TNF inhibitors will still be first-line in 2028, even as JAK and IL-17 drugs grow. Why? Because their long-term cancer safety data is better.

What Should You Do?
Don’t panic. Don’t stop your drug without talking to your doctor. But do be smart.
- Get age-appropriate cancer screenings before starting a TNF inhibitor - mammograms, colonoscopies, skin checks.
- If you’ve had skin cancer, ask for a dermatology referral. Get checked every six months.
- If you’ve had another cancer, make sure your rheumatologist and oncologist talk to each other. Don’t assume they will - make it happen.
- Ask: “Is etanercept an option for me?” It may carry less skin cancer risk than adalimumab.
- Reduce steroid use if you can. Even cutting prednisone from 10 mg to 5 mg makes a difference.
- Don’t ignore symptoms. A new mole, unexplained weight loss, or persistent cough? Tell your doctor. Early detection saves lives.
Most patients who worry about cancer risk end up staying on TNF inhibitors - and they’re glad they did. A 2023 National Psoriasis Foundation survey found 78% of patients would restart their TNF inhibitor after cancer treatment. Why? Because they got their life back.
The Bottom Line
The fear of cancer from TNF inhibitors is real - but mostly overblown. The data doesn’t support a broad increase in cancer risk. If anything, the risk is small, specific, and manageable.
Adalimumab has a slight signal for early cancer diagnosis - likely due to underlying disease, not the drug. Etanercept looks safer for skin cancer. Steroids are riskier than biologics. And for many, the benefit of living without constant pain, fatigue, and disability far outweighs the tiny, measurable risk.
What matters most isn’t the drug name. It’s your history, your age, your cancer screening, and your team. A good rheumatologist doesn’t just prescribe a drug. They help you weigh the numbers, listen to your fears, and make a plan that fits your life.
You’re not choosing between safety and treatment. You’re choosing between better health and a very small, manageable risk. And for most people, that’s an easy choice.



John Ross
4 January 2026 - 04:57 AM
TNF inhibitors aren't some magical cure-all - they're immunomodulators with off-target effects that are still being mapped. The real issue isn't cancer risk per se, it's the heterogeneity in patient response and the confounding variables in observational data. Monoclonal antibodies like adalimumab bind TNF with high affinity and prolonged half-life, potentially disrupting immune surveillance in lymphoid tissues. Etanercept, being a soluble receptor fusion, has faster dissociation kinetics - which may explain its cleaner safety profile. We need longitudinal proteomic and T-cell receptor sequencing studies to truly understand the mechanistic divergence.
Clint Moser
5 January 2026 - 22:11 PM
theyre hiding the truth again… big pharma paid off the swedish registry… etanercept is safe because it was made by a company that also makes vaccines… and adalimumab? thats the one they push because its patent expires last… the cancer stats are manipulated… i know people who got lymphoma after 6 months… no one talks about it
Aaron Mercado
6 January 2026 - 06:58 AM
Let me be perfectly clear: if you're taking a biologic and you haven't had a full-body dermatological exam, a colonoscopy, and a mammogram - you're not being responsible, you're being reckless. And if your doctor didn't explicitly tell you to do all three before prescribing? They're negligent. This isn't a game. You're playing Russian roulette with your immune system and calling it 'medical progress.' I've seen too many people die from 'early-stage' cancers that were missed because they trusted the hype. Stop being lazy. Get screened. Now.
Vikram Sujay
7 January 2026 - 17:32 PM
The discourse surrounding TNF inhibitors often lacks a nuanced appreciation for the dialectic between inflammation and carcinogenesis. While immunosuppression is a legitimate concern, the chronic inflammatory milieu inherent in autoimmune conditions may itself be a more potent oncogenic driver than the pharmacological intervention. The observed correlation between adalimumab and early cancer diagnoses may reflect diagnostic vigilance rather than etiological causation. One must consider the epistemological limitations of registry data and the potential for ascertainment bias. A holistic view, integrating molecular biology, epidemiology, and patient narrative, is imperative.
Jay Tejada
9 January 2026 - 06:55 AM
my buddy took adalimumab for 3 years, got a basal cell removed last year - no big deal. he got it checked every 6 months like the article said. still plays basketball. still works. still alive. maybe the real risk is not taking the drug and spending your life in pain? just saying.
Shanna Sung
11 January 2026 - 04:46 AM
they're lying. every single one of them. the FDA knows etanercept causes melanoma but they let it slide because it's cheaper. they're putting profits over people. i know a woman who died after 8 months on it. her skin turned black. no one wants to talk about it because they're scared of the lawsuit. you think this is medicine? it's corporate murder with a stethoscope
Allen Ye
12 January 2026 - 18:57 PM
Let’s not reduce this to a binary choice between safety and efficacy. The question isn’t whether TNF inhibitors cause cancer - it’s whether they tip the balance of a pre-existing pathological state toward malignancy. The immune system doesn’t just guard against tumors; it sculpts them through immunoediting. TNF is a double-edged sword: it promotes inflammation, yes, but it also activates dendritic cells, enhances T-cell priming, and induces apoptosis in malignant clones. The differential effects between monoclonal antibodies and fusion proteins may lie in their spatial-temporal modulation of TNF bioavailability - not just binding affinity. We’re not just treating arthritis; we’re altering the tumor microenvironment. That’s why etanercept’s lower skin cancer risk isn’t an accident - it’s a pharmacodynamic signature. We need more head-to-head proteomic studies, not just registry re-analyses.
mark etang
14 January 2026 - 04:18 AM
As a healthcare professional with over two decades of clinical experience, I can unequivocally state that the benefits of TNF inhibitors in appropriately selected patients vastly outweigh the risks. The data is robust, the guidelines are evidence-based, and the outcomes are transformative. Patients who avoid these therapies due to unfounded fear often experience irreversible joint destruction, loss of employment, and profound disability. We must prioritize patient education, informed consent, and structured monitoring protocols - not alarmism. The medical community has a duty to uphold scientific integrity over sensationalism.
josh plum
15 January 2026 - 19:54 PM
you people are so naive. you think the FDA gives a damn about your skin cancer? they care about stock prices. adalimumab made $20 billion last year. etanercept? barely a billion. guess which one they're pushing in every ad? the one that makes them the most money. and don't even get me started on the biosimilars - they're not the same, trust me, i've seen the batches. if you're on this stuff, you're basically a lab rat for Big Pharma. and you're okay with that? i'm not.
Brendan F. Cochran
15 January 2026 - 20:12 PM
USA first! we don't need no foreign studies to tell us what's safe. sweden's got worse cancer rates than us anyway. and that reddit survey? that's just a bunch of whiny kids. if you're american and you got arthritis, you take what the doc says and you shut up. we got better medicine here than those europeans. etanercept? nah, adalimumab is the real deal. if you got skin cancer? good. now you know to stay outta the sun. problem solved.
jigisha Patel
15 January 2026 - 23:09 PM
The statistical interpretation presented is fundamentally flawed. The hazard ratio of 0.98 for overall cancer risk ignores subgroup analyses, competing risks, and time-varying exposure. The apparent protective effect of etanercept may be attributable to confounding by indication - patients prescribed etanercept are often younger, healthier, and have lower disease burden. Furthermore, the 22% reduction in cancer incidence lacks adjustment for smoking, BMI, and prior immunosuppressant use. Without multivariate Cox regression with inverse probability weighting, these claims are speculative at best. The data does not support the conclusion drawn.
Jason Stafford
17 January 2026 - 04:42 AM
they're all lying. the real cancer risk is 10x higher than they say. they don't tell you about the hidden deaths - people who die from lymphoma but it's listed as 'complications of RA.' they bury the data. i've got a spreadsheet of 47 cases from 2018 to 2023. every single one was on a biologic. and guess what? they all started with a rash. they all ignored it. now they're all gone. you think you're safe? you're not. you're one mole away from being a statistic.
Mandy Kowitz
18 January 2026 - 05:54 AM
so let me get this straight - you're telling me the drug that costs $62k a year is safer than prednisone? and i'm supposed to be grateful? i'm on both. i'm also on a waiting list for a liver transplant because i took 10mg of prednisone for 12 years. so yeah. thanks for the 'safer' option. i'll stick with my $10 methotrexate and my chronic pain. at least i'm not bankrupt.
Justin Lowans
19 January 2026 - 05:39 AM
What strikes me most is the profound asymmetry between public perception and clinical reality. The fear of cancer, while understandable, often eclipses the lived experience of uncontrolled inflammation - the fatigue that steals sleep, the joints that refuse to bend, the loss of identity that comes with chronic disability. The data, when properly contextualized, reveals not a monster in the form of a biologic, but a tool - imperfect, yes, but capable of restoring agency. The true risk lies not in the molecule, but in the silence that follows fear - the patient who stops treatment, the doctor who doesn't listen, the system that fails to provide screening. We must speak not only of molecules, but of meaning.
Cassie Tynan
19 January 2026 - 13:06 PM
the fact that we're even having this conversation is insane. we're treating chronic pain with lab-made proteins while ignoring sleep, diet, and stress. but sure, let's blame the drug. i've been on etanercept for 7 years. got two basal cells removed. both were tiny. i got them checked. i'm fine. but i also meditate, eat veggies, and don't sit all day. maybe the real miracle isn't the drug - it's that we still think a pill can fix everything.