Boxed Warning Impact Calculator
How Specific is Your Drug Warning?
Based on FDA's 2025 requirements, warnings with clear data and actions lead to significant prescription changes. This tool estimates the impact.
Every year, the FDA updates the most serious safety alerts on prescription drugs-those bold, black-bordered warnings you might have seen on pill bottles or in electronic health records. These aren’t just fine print. They’re life-or-death signals. In 2025, 47 new or revised boxed warnings were issued, the highest number since 2020. That’s not noise. It’s a shift in how we understand drug risks-and how those risks change what doctors prescribe, what pharmacists check, and what patients actually take.
What Exactly Is a Boxed Warning?
A boxed warning, also called a black box warning, is the strongest safety alert the FDA can require. It’s not a suggestion. It’s a legal requirement. If a drug carries one, it means clinical evidence shows a real risk of serious injury, permanent disability, or death. These warnings appear at the very top of a drug’s prescribing information, surrounded by a thick black border to make sure no one misses them. They’re not for minor side effects like headaches or nausea. They’re for things like: liver failure from certain seizure meds, sudden heart rhythm changes from antibiotics, or fatal breathing problems if an opioid patch is given to someone who’s never taken opioids before. The FDA started using them in the 1970s, but their use has exploded in the last decade. Today, over 400 prescription drugs in the U.S. carry one. That’s about one in eight of all medications on the market.What Changed in 2025?
The biggest shift in 2025 wasn’t just the number of new warnings-it was the specificity. For years, many boxed warnings were vague. Phrases like “monitor for hepatotoxicity” or “risk of serious infection” were common. But starting January 2024, the FDA began requiring all new or revised warnings to include hard numbers and clear actions. For example:- Instead of “may cause myocarditis,” you now see: “1.2% incidence of myocarditis in patients under 30, with highest risk within 7 days of first dose.”
- Instead of “avoid in renal impairment,” you now see: “Do not use if eGFR is below 30 mL/min. Reduce dose by 50% if eGFR is 30-50 mL/min.”
- For GLP-1 agonists like semaglutide, the new warning states: “Risk of gallbladder disease increases by 45% after 6 months of use; discontinue if persistent abdominal pain occurs.”
Why This Matters for Doctors and Pharmacists
For prescribers, this isn’t just about reading a warning. It’s about changing behavior. A 2020 study in the New England Journal of Medicine found that warnings with specific, measurable instructions were 3.2 times more likely to change how doctors prescribe than vague ones. Take clozapine, an antipsychotic with a boxed warning for agranulocytosis-a dangerous drop in white blood cells. Since 2015, the warning required absolute neutrophil count (ANC) checks every week for the first 6 months. But in 2025, the FDA added: “Discontinue if ANC falls below 1,000/mm³ and do not restart unless ANC recovers above 1,500/mm³ and patient is monitored daily for 3 days.” That’s not just a reminder. It’s a protocol. Pharmacists are now required to verify these details before dispensing. At hospitals like Henry Mayo Newhall, pharmacists must confirm opioid tolerance before releasing fentanyl patches. They check if the patient has a documented history of opioid use. If not? The prescription gets flagged. The system isn’t perfect-EHR alerts often trigger too many false alarms-but the rules are getting tighter.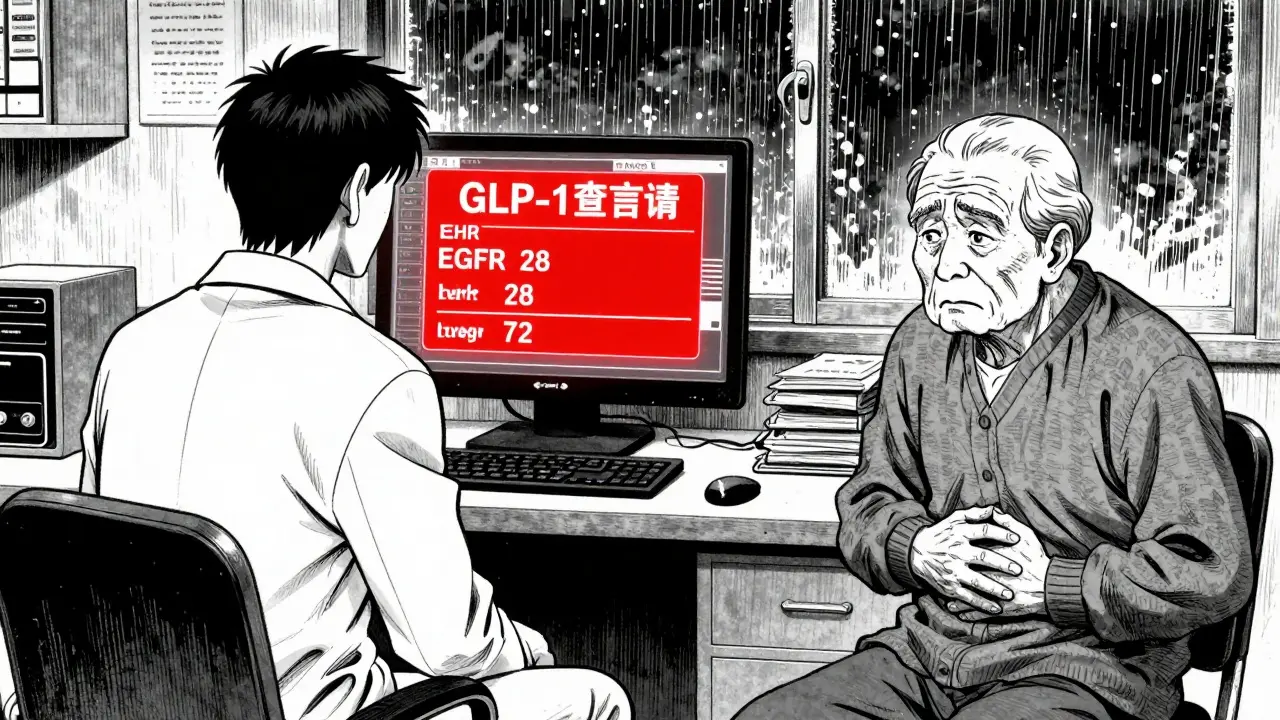
How Patients Are Affected
Patients don’t always see these warnings. But they feel their impact. Some medications are now harder to get. A 2021 study showed that when a drug gets a new boxed warning, prescriptions drop by 15% to 70%, depending on the drug and how much media attention it gets. Rosiglitazone’s warning about heart risks caused a 70% drop in prescriptions-even though it still helped some patients. Meanwhile, pioglitazone, with a similar warning, saw little change because it didn’t make headlines. But for others, the warnings are lifesaving. Isotretinoin (Accutane), which can cause severe birth defects, requires enrollment in the iPledge program. Patients must take pregnancy tests, use two forms of birth control, and sign paperwork. A 2021 FDA patient forum found that 78% of users said this clear system made them more likely to follow the rules. The problem? Many patients don’t understand the warnings. A Medscape survey found that 44% of doctors believe boxed warnings sometimes delay urgent care-especially in emergencies. For example, a patient with sepsis might need moxifloxacin, which carries a QT prolongation warning. Doctors hesitate. But if they don’t give the antibiotic, the patient could die. The warning isn’t wrong-it’s just not always easy to apply in real time.What’s Coming Next
The FDA isn’t slowing down. Its 2023-2027 plan aims to issue 25% more boxed warnings based on real-world evidence by 2027. The focus? Long-term risks of newer drugs. GLP-1 agonists (like Ozempic and Wegovy) are under heavy scrutiny. Early data shows increased risks of pancreatitis, gastroparesis, and gallbladder disease after 6-12 months of use. Immune checkpoint inhibitors for cancer are also being watched for rare but deadly autoimmune reactions that can show up months after treatment ends. And there’s a new pilot program testing “dynamic” warnings. In 15 health systems, EHR alerts now adjust based on patient data. If you’re 72 with kidney disease, the alert for a drug like metformin will say: “High risk of lactic acidosis. Avoid if eGFR <30.” If you’re 25 and healthy? The alert disappears. That’s cutting down alert fatigue by 37% in early trials.
Where the System Still Falls Short
Despite progress, problems remain. A 2022 GAO report found that 31% of boxed warnings still lack enough detail to guide real decisions. Some still say “monitor liver function” without saying how often, what test, or what level is dangerous. Another issue? Overuse. NSAIDs like ibuprofen carry a boxed warning for gastrointestinal bleeding. But since almost everyone takes them, the warning has become background noise. A 2021 AMA survey found that 52% of doctors say these warnings are “too common” to be meaningful. And then there’s the human factor. On Sermo, a physician forum, 73% of doctors said they routinely override boxed warnings for palliative care patients. Why? Because sometimes the risk of not treating is worse than the risk of treating. A dying patient with severe pain doesn’t need to wait for a 10-step safety checklist.What You Should Do
If you’re on a prescription:- Ask your pharmacist: “Does this drug have a black box warning? What does it mean for me?”
- Ask your doctor: “Is this warning based on real data from people like me?”
- Check your EHR portal. Many now list boxed warnings in the medication summary.
- If you’re on a drug with a new warning, ask about monitoring: “Do I need blood tests? How often? What numbers should I watch?”
- Keep a list of all medications with boxed warnings.
- Know the red flags: sudden chest pain, unexplained bruising, jaundice, severe abdominal pain, confusion.
- Don’t assume the doctor knows everything. Bring the warning label to appointments.
Final Thoughts
Boxed warnings aren’t meant to scare you off medicine. They’re meant to help you use it safely. The changes in 2025 make them more useful than ever-more precise, more data-driven, more actionable. But they’re only as good as the systems that deliver them and the people who understand them. The goal isn’t to stop prescribing. It’s to prescribe smarter. To know when the risk is worth it. To catch problems before they happen. And to make sure that when a drug says “this could kill you,” it’s not just a warning on a page-it’s a conversation that saves lives.What does a boxed warning mean on a prescription drug?
A boxed warning, also called a black box warning, is the strongest safety alert the FDA requires. It means clinical evidence shows the drug can cause serious, life-threatening, or even fatal side effects. These warnings appear at the top of the drug’s prescribing information and are designed to ensure prescribers understand and manage the risks before giving the medication.
How many drugs have boxed warnings in 2025?
As of 2025, over 400 prescription drugs in the U.S. carry active boxed warnings. That’s about 12% of all FDA-approved medications. The number has been rising each year, with 47 new or revised warnings issued in 2025 alone-up from 42 in 2024.
Why are boxed warnings changing in 2025?
In 2024, the FDA began requiring all new or revised boxed warnings to include specific, quantified data. Instead of vague statements like “monitor for liver damage,” warnings now must say exactly what test to run, how often, and what result triggers action. For example: “Obtain ALT/AST levels at baseline and monthly for the first 6 months. Discontinue if levels rise above 3x the upper limit of normal.” This change was made because older warnings were too general to guide real clinical decisions.
Do boxed warnings actually reduce harm?
Yes, but not always. The FDA estimates that since 2015, boxed warnings have prevented about 12,000 serious adverse events each year. However, effectiveness depends on specificity. Warnings with clear monitoring steps are 3.2 times more likely to change prescribing behavior than vague ones. Some warnings, especially for common drugs like NSAIDs, have lost impact due to overuse and alert fatigue.
Can I still take a drug with a boxed warning?
Yes, if the benefits outweigh the risks. Many drugs with boxed warnings-like warfarin, clozapine, or chemotherapy agents-are still essential. The warning doesn’t mean “don’t use it.” It means “use it carefully.” Your doctor will consider your health history, monitor you closely, and may require blood tests or other checks to reduce risk. Never stop a medication without talking to your provider.
What should I do if I see a boxed warning on my medication?
Don’t panic. Ask three questions: 1) What is the specific risk? 2) How will my doctor monitor for it? 3) Are there alternatives? Get the details in writing. Check your electronic health record for the warning. Keep a list of all your medications with boxed warnings and bring it to every appointment. If you notice symptoms like jaundice, chest pain, or unusual bruising, contact your provider immediately.
Are boxed warnings the same worldwide?
No. The U.S. FDA uses the black box format, but other countries have different systems. The European Medicines Agency uses “contraindications” and “warnings” without the black border. Canada and Australia have similar but less standardized systems. The FDA’s approach is the most formalized and legally enforceable. A drug with a boxed warning in the U.S. may not have the same alert elsewhere.
Why do some drugs get boxed warnings years after approval?
Many serious side effects only show up after thousands of people use the drug for months or years. Clinical trials usually involve a few thousand patients over a few months. Real-world use reveals rare or long-term risks. The FDA uses systems like FAERS (Adverse Event Reporting System) and the Sentinel Initiative to monitor millions of patient records and detect patterns. That’s why drugs like Vioxx (withdrawn for heart risks) or GLP-1 agonists (now being watched for gallbladder disease) get warnings years after launch.





jaspreet sandhu
2 January 2026 - 05:12 AM
they keep adding these warnings like its some kind of game. i seen a guy on reddit say his grandma got denied metformin cause her eGFR was 32 and she was 87 and walked 3 miles a day. what the actual f. theyre not protecting people theyre just covering their asses with more black boxes.
Alex Warden
3 January 2026 - 20:00 PM
USA still leads the world in drug safety. Other countries? They let people die with no warnings. We got data, we got science, we got accountability. If you cant handle a black box warning you probably shouldnt be taking pills anyway. #AmericanMedicine
Bryan Anderson
5 January 2026 - 02:49 AM
The shift toward quantified risk in boxed warnings is a significant improvement in clinical communication. Earlier vague language led to inconsistent application across providers. Now, with specific thresholds like eGFR cutoffs and incidence percentages, prescribers can make more informed decisions. This reduces both under-treatment and over-caution. It's a step toward precision medicine in real-world practice.
Matthew Hekmatniaz
5 January 2026 - 16:00 PM
It’s good to see the FDA finally listening to real-world data instead of just lab studies. But we also need to remember that not everyone has access to EHR alerts or pharmacists who double-check prescriptions. In rural areas or low-income clinics, these warnings might just mean a patient gets denied meds without any explanation. We can’t just make the system smarter-we need to make it fair too.
Liam George
6 January 2026 - 02:19 AM
you ever wonder why the FDA suddenly cares about ‘real-world evidence’ in 2024? big pharma paid them off. the sentinel initiative? funded by Merck and Novo Nordisk. they knew GLP-1s would cause gallbladder issues but waited until they were on every corner store shelf. now they slap a warning on it and act like heroes. the same companies that pushed opioids for years are now the ones ‘saving’ us from their own drugs. it’s all a play. the black box isn’t a warning-it’s a liability shield.
Dusty Weeks
6 January 2026 - 04:13 AM
so like… if u got a black box warning does that mean ur pill is cursed? 🤔💀 i just want my zoloft to work without some gov’t guy in a lab telling me i might die if i take it with coffee
Sally Denham-Vaughan
6 January 2026 - 13:14 PM
my aunt got prescribed semaglutide for diabetes and ended up in the ER with gallbladder pain. she didn’t even know the warning existed until the pharmacist called her. they just hand you the script and say ‘take one daily.’ no one explains the 45% risk. it’s not the warning’s fault-it’s the system that doesn’t bother to talk to patients.
Richard Thomas
6 January 2026 - 20:34 PM
Every time we add another black box, we’re not just warning-we’re shaping culture. We’re teaching people to fear medicine instead of trust it. The body is not a machine with error codes. It’s a living system that adapts, fails, and sometimes heals. When we reduce human health to percentages and thresholds, we lose the art of care. A doctor used to look at a patient, not just an eGFR number. Now we’re training them to be data clerks with stethoscopes. Is this progress-or just efficiency dressed up as safety?
Paul Ong
7 January 2026 - 15:10 PM
the system works better now but its still broken. if you dont have a good pharmacist or a doctor who listens you get left behind. i seen too many people just stop taking meds cause they got scared by a black box and no one explained it. we need more education not more borders