When your doctor prescribes a medication, you might assume the brand-name version is the only option. But in reality, over 90% of prescriptions in the U.S. are filled with generic medications-saving billions each year. However, the question remains: is switching to generics always safe? Clinical studies provide answers, but the picture isn’t black and white. Let’s break down what the research really says about brand-to-generic switches.
How Generic Medications Are Approved
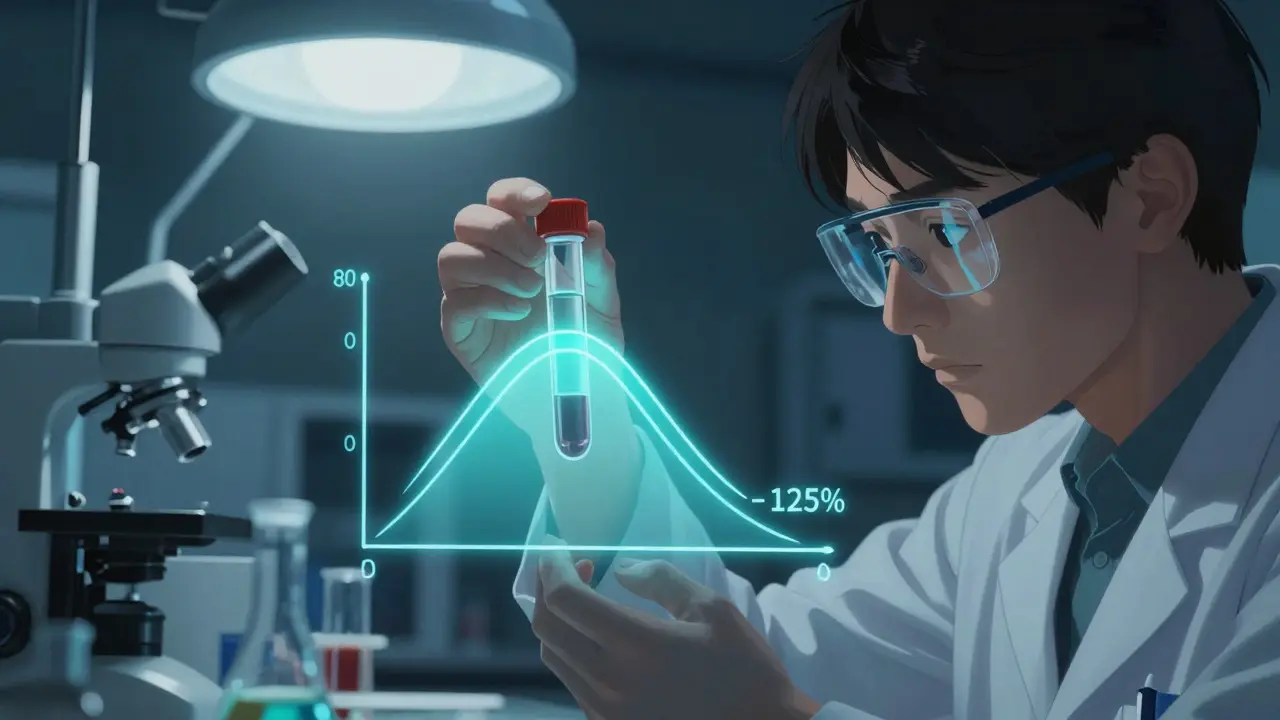
FDA requires generic drugs to demonstrate bioequivalence, meaning they must deliver the same amount of active ingredient into the bloodstream within the same timeframe as the brand-name drug. This standard uses a 90% confidence interval between 80% and 125% of the brand’s pharmacokinetic measurements. For example, if a brand-name drug delivers 100 units of active ingredient, the generic must deliver between 80 and 125 units. The FDA’s Orange Book shows this rigorous testing covers over 90% of prescriptions in the U.S., saving $370 billion annually.
Why Some Switches Cause Problems
Not all medications are created equal when it comes to switching. Drugs with a narrow therapeutic index (NTI) are especially tricky. These are medications where small changes in blood concentration can cause serious side effects or treatment failure. Think of it like a tightrope walk: too little and the drug doesn’t work; too much and it’s toxic. Antiepileptic drugs and certain heart medications fall into this category. For instance, generic phenytoin studies show 22%-31% lower plasma drug levels compared to brand-name versions, directly impacting seizure control.
Antiepileptic Drugs: A Closer Look
Research on antiepileptic drugs reveals clear risks. A 2017 review of 760 epileptic patients found generic levetiracetam caused blurred vision in 18.7% of cases, headaches in 24.3%, and mood swings in 11.4%. Some patients even needed to switch back to brand-name drugs due to increased seizures. Another study tracked 50 patients who were stable on brand-name antiepileptics but experienced breakthrough seizures after switching to generics. Nearly half had lower drug levels in their blood at the time of the seizures. The American Academy of Neurology acknowledges these risks, advising doctors to monitor patients closely after any switch.

Cardiovascular Medications: Mixed Results
For heart medications, the story is more nuanced. A 2020 Nature Scientific Reports study analyzed 8.5 million Austrians’ health records and found generics for most cardiovascular drugs actually had better outcomes. Simvastatin generics reduced death risk by 22%, while atorvastatin generics cut major cardiac events by 15%. However, exceptions exist: bisoprolol and nebivolol generics showed worse results in some studies. A separate 2017 Circulation study also found a 12.3% higher rate of side effects for generic amlodipine (a blood pressure drug) compared to its brand-name counterpart. The key takeaway? Not all heart medications behave the same when switched to generics.
Expert Perspectives on Switching
Doctors have nuanced views on generic switches. Dr. Choudhry from Harvard Health noted, “The existing data are reassuring for most drugs,” but emphasized small studies can’t capture every patient’s experience. Dr. Tsourounis from UCSF warns patients not to jump to conclusions about efficacy differences without medical consultation. The FDA’s 2023 draft guidance now specifically addresses NTI drugs like antiepileptics, requiring additional bioequivalence studies. Meanwhile, the European Medicines Agency advises special attention for patients with unstable epilepsy, multiple medications, or conditions affecting drug metabolism.

What Patients Should Know
Real-world switching patterns reveal critical gaps. A 2023 study tracking 218 patients found only 19.7% knew what their medication was for, while 67% identified generics by color or shape. This led to 11.5% of patients accidentally taking duplicate medications. For those with chronic conditions like epilepsy, switching back from generic to brand happened in 12.8% of cases. The cost of managing seizures after a generic switch averages $1,850 per incident-far outweighing the initial savings. Always discuss switching with your doctor and pharmacist. Ask if your medication has a narrow therapeutic index and whether therapeutic drug monitoring is needed.
Current Research and Future Directions
Ongoing studies are filling critical knowledge gaps. A 2022 JAMA Neurology study of 112,456 epilepsy patients found generic switches initially increased emergency room visits by 12.7%, but this risk faded after 90 days. Researchers now focus on genetic factors: a 2023 University of Toronto study identified specific enzyme variations (CYP2C9 and CYP2C19) that make some patients more vulnerable to concentration changes between brands and generics. The FDA’s Sentinel Initiative now tracks 15 million patient records for adverse events after generic substitution, especially in critical drug classes. This data will shape future guidelines to balance cost savings with patient safety.
Comparison of Generic vs. Brand Medication Outcomes by Drug Class
| Drug Class | Generic Performance | Key Findings |
|---|---|---|
| Antiepileptic Drugs | Variable | Generic levetiracetam: 18.7% blurred vision, 24.3% headaches. Generic phenytoin: 22%-31% lower plasma drug levels. |
| Cardiovascular Medications | Generally Positive | Simvastatin: 22% lower death risk with generics. Atorvastatin: 15% fewer cardiac events. Exceptions: bisoprolol and nebivolol showed worse outcomes. |
| Blood Pressure Medications | Mixed | Generic amlodipine: 12.3% higher adverse event rates. Branded medications had 26.7% higher discontinuation rates. |
Are generic medications always safe to switch to?
Most generic medications are safe and effective substitutes for brand-name drugs, but certain classes require caution. The FDA requires bioequivalence testing, which ensures generics deliver the same active ingredient within 80%-125% of the brand’s levels. However, drugs with narrow therapeutic indices (NTIs), like some antiepileptics and cardiovascular medications, may have clinically significant differences. For example, generic phenytoin showed 22%-31% lower plasma drug levels in some patients, leading to seizure control issues. Always consult your doctor before switching.
Which medications have higher risks when switching to generics?
Antiepileptic drugs like phenytoin and levetiracetam carry the highest risks, with studies showing lower drug levels and increased seizures after switching. Certain blood pressure medications like amlodipine and heart drugs like bisoprolol also show inconsistent results. Medications with narrow therapeutic indices (NTIs) are the main concern-these are drugs where small changes in blood concentration can cause serious harm. Your pharmacist can check if your medication falls into this category.
Should I avoid generic medications entirely?
No. For most medications, generics are just as safe and effective as brand-name versions. The FDA’s rigorous testing process ensures quality for the vast majority of drugs. Generic medications account for over 90% of prescriptions in the U.S. and save the healthcare system $370 billion yearly. Avoiding generics entirely would mean missing out on significant cost savings without medical justification. The key is to discuss your specific medication with your doctor to determine if switching is appropriate for your situation.
How can I tell if my medication has a narrow therapeutic index?
Ask your pharmacist or doctor directly. Medications with narrow therapeutic indices typically include antiepileptics (like phenytoin or carbamazepine), blood thinners (like warfarin), certain heart medications (like digoxin), and some immunosuppressants (like cyclosporine). These drugs often require regular blood tests to monitor levels. If your medication falls into this category, your healthcare provider will likely recommend sticking with the same manufacturer’s version to avoid fluctuations in effectiveness.
What should I do if I experience side effects after switching to a generic?
Contact your doctor immediately. Document any symptoms, including when they started and how severe they are. Your doctor may order blood tests to check drug levels or suggest switching back to the brand-name version. In some cases, the issue may resolve after a few weeks as your body adjusts. However, for drugs with narrow therapeutic indices, persistent side effects could indicate a problem. Never stop taking medication without consulting your healthcare provider, as this could lead to serious health consequences.




Mark Harris
7 February 2026 - 13:33 PM
Hey folks! So I've been reading up on this generic meds switch thing. It's pretty interesting.
Like, the FDA requires generics to be bioequivalent, which means they have to deliver the same active ingredient within 80-125% of the brand.
That's pretty tight.
But for drugs with narrow therapeutic index, like some epilepsy meds, even small changes can cause issues.
For example, generic phenytoin has shown 22-31% lower plasma levels in some studies.
That's a big deal for seizure control.
And for heart meds, it's mixed.
Some generics like simvastatin actually have better outcomes, but others like bisoprolol can be problematic.
The key is to know your specific medication.
If you're on something like warfarin or digoxin, you need to be careful.
Always talk to your doctor before switching.
They can check if your drug has a narrow therapeutic index.
And if you do switch, monitor your symptoms closely.
I've had friends who had issues with generic switches, but others who didn't.
It really varies.
But overall, generics save a ton of money, which is great for the healthcare system.
Just need to be smart about it.
Don't just assume all generics are the same.
Knowledge is power here.
Stay safe!
Catherine Wybourne
7 February 2026 - 15:08 PM
True that. In the UK we've had similar debates. Though the NHS is pretty strict on generics. But honestly, it's a bit of a gamble sometimes. 😏
Ashley Hutchins
8 February 2026 - 14:22 PM
Generics are fine for most but for epilepsy patients its a disaster Always go brand name No exceptions Seriously people need to wake up
Niel Amstrong Stein
9 February 2026 - 20:41 PM
NHS is strict but even there some generics have issues. I read a study where they had to switch back for some patients. 🤔
Paula Sa
10 February 2026 - 06:04 AM
It's all about the individual case. Some people do great with generics, others need the brand. Doctors should monitor closely. Just saying.
Mary Carroll Allen
10 February 2026 - 10:14 AM
OMG yes! My friend had a seizure after switching. So scary. Always check with your pharmacist. Like seriously, do it.
Joey Gianvincenzi
11 February 2026 - 08:51 AM
The FDA's stringent testing protocol ensures that generic medications meet the highest standards of safety and efficacy. Any deviation from this is unacceptable.
Amit Jain
11 February 2026 - 23:57 PM
Wait, the FDA's testing is crap. I've seen way too many bad reactions. It's all about the money. Big pharma doesn't want generics to work.
Heather Burrows
13 February 2026 - 05:23 AM
Yeah, but you're just being paranoid. Most generics are fine. Like, seriously, don't be so dramatic.
Ritu Singh
14 February 2026 - 20:05 PM
While it's important to be cautious, it's also vital to remember that generics are essential for global healthcare access. We must balance safety with affordability.
Sarah B
16 February 2026 - 17:20 PM
America makes the best meds period
Eric Knobelspiesse
17 February 2026 - 23:11 PM
Generic switch issues are overhyped. Most studies show no difference. But yeah, maybe for some niche drugs. But overall, it's fine.